
Why? Because these are the electrons that are the least tightly held. The pair of electrons on the nucleophile almost always comes from the highest-energy occupied molecular orbital (HOMO) of the nucleophile.In order for that bond to form, the filled orbital on the nucleophile containing the electron pair has to come into contact (overlap) with the empty orbital on the electrophile which can accept the electron pair. Most reactions we’ve seen involve a nucleophile (an electron-pair donor) reacting with an electrophile (an electron-pair acceptor) to form ONE new bond.
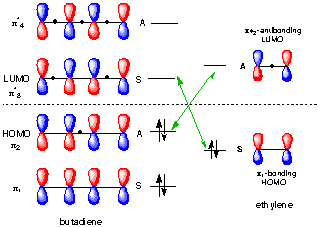
Bond Formation Requires Overlap Between The HOMO Of One Molecule (The Nucleophile) With The LUMO Of Another Molecule (The Electrophile) We will scratch the surface of the orbital symmetry rules here and use them to show why the reaction of dienes with alkenes (the Diels-Alder) occurs readily upon heating, but the reaction of alkenes with alkenes (a.k.a. In the 1960’s a theory of orbital symmetry was developed to understand these reactions, which provided a very useful set of predictive rules – the Woodward-Hoffmann rules. The answer to this question lies in the arrangement of pi molecular orbitals in the two components of this reaction. Why is the Diels-Alder so easy, and many seemingly related reactions so hard? Nor do two dienes combine easily upon heating to give eight-membered rings. After all, we’ve seen plenty of examples of things that don’t work two alkenes, for example, don’t combine to form four membered rings upon heating in the way that a diene and a dienophile combine to form a six-membered ring. What we haven’t really covered is why the Diels-Alder actually works. Generally the endo– is favored over the exo.
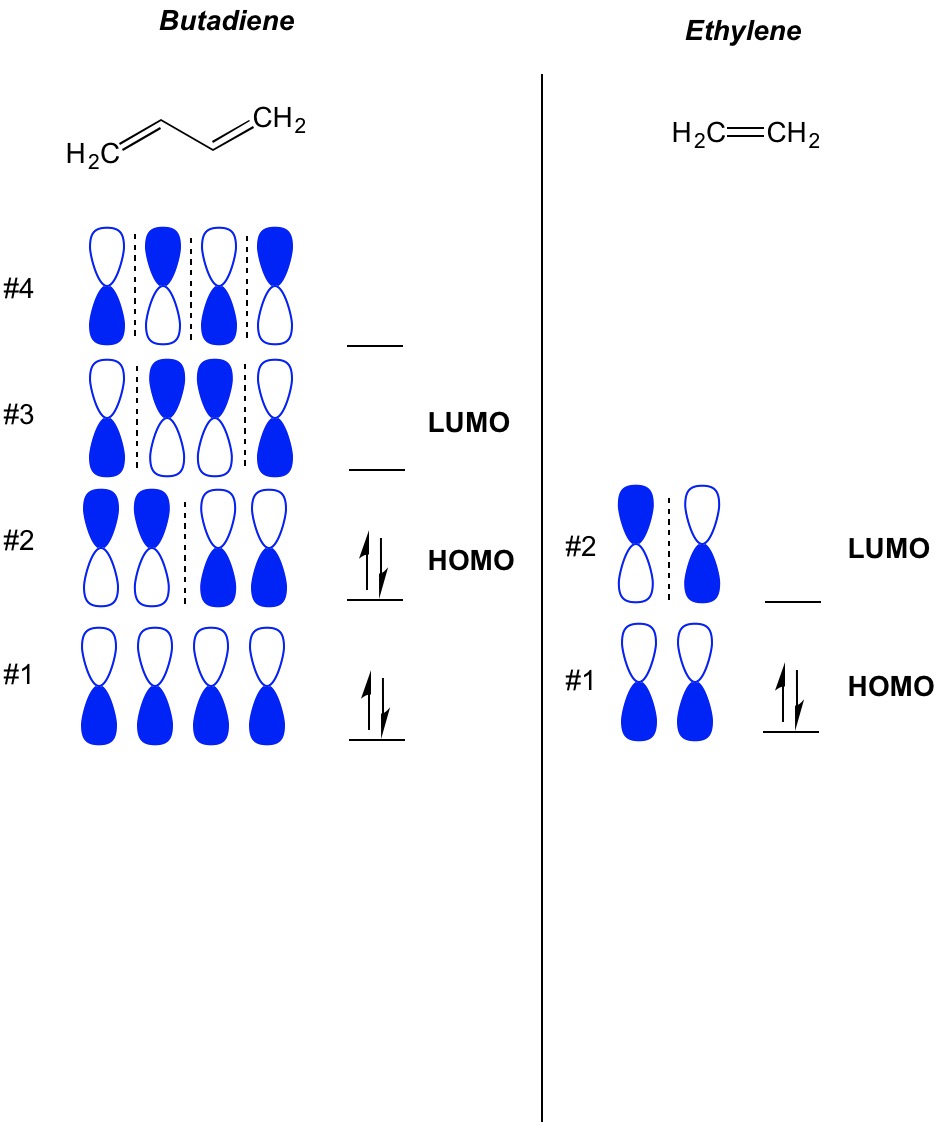
Under “Photochemical” Conditions, The Actually Works Pretty Well.Molecular Orbitals In The Diels-Alder Reaction: Interaction of the Diene HOMO with the Dienophile LUMO Is Favorable At Both Bond-Forming Sites.Molecular Orbitals In The Cycloaddition Between Ethene And Ethene Show Why The Reaction Is Unfavorable Under “Thermal” Conditions.Concerted Reactions: When Two Bonds Form At The Same Time, Multiple Orbitals Must Overlap.Bond Formation Requires Overlap Between The HOMO Of One Molecule (The Nucleophile) With The LUMO Of Another Molecule (The Electrophile).A Quick Recap Of The Diels-Alder Reaction.
#Lumo and humo how to
Using our previous posts on how to build up molecular orbitals, we’ll show how the Diels-Alder results from the constructive orbital overlap between the highest-occupied molecular orbital (HOMO) of the diene with the lowest-unoccupied molecular orbital (LUMO) of the dienophile. Today we’re going to go into the mechanism of the Diels-Alder reaction from a molecular orbital perspective. The HOMO and LUMO In The Diels Alder Reaction


 0 kommentar(er)
0 kommentar(er)
